Ionization energy: 1st
The 1st ionization energy of the element M is a measure of the energy required to remove one electron from one mole of the gaseous atoms M
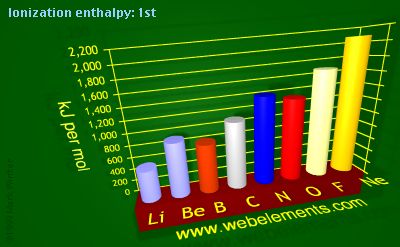
Units
kJ mol-1
Notes
None
For access to other ionisation enrgies, select from:
- Ionisation energy definition
- 1st ionisation energies
- 2nd ionisation energies
- 3rd ionisation energies
- 4th ionisation energies
- 5th ionisation energies
- 6th ionisation energies
- 7th ionisation energies
- 8th ionisation energies
- 9th ionisation energies
- 10th ionisation energies
- 11th ionisation energies
- 12th ionisation energies
- 13th ionisation energies
- 14th ionisation energies
- 15th ionisation energies
- 16th ionisation energies
- 17th ionisation energies
- 18th ionisation energies
- 19th ionisation energies
- 20th ionisation energies
- 21st ionisation energies
Literature sources
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry: Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
- D.R. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 79th edition, 1998.
- J.A. Dean (ed) in Lange's Handbook of Chemistry, McGraw-Hill, New York, USA, 14th edition, 1992.
- A. Kramida, Yu. Ralchenko, J. Reader, and NIST ASD Team (2018). NIST Atomic Spectra Database (ver. 5.5.6), [Online]. Available: https://physics.nist.gov/asd [Accessed 10 March 2018]. National Institute of Standards and Technology, Gaithersburg, MD.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||