Arsenic - 33As: the essentials
- Name: arsenic
- Symbol: As
- Atomic number: 33
- Relative atomic mass (Ar): 74.921595 (6)
- Standard state: solid at 298 K
- Appearance: metallic grey
- Classification: Semi-metallic
- Group in periodic table: 15
- Group name: Pnictogen
- Period in periodic table: 4
- Block in periodic table: p
- Shell structure: 2.8.18.5
- CAS Registry: 7440-38-2
Arsenic atoms have 33 electrons and the shell structure is 2.8.18.5. The ground state electronic configuration of neutral arsenic is [Ar].3d10.4s2.4p3 and the term symbol of arsenic is 4S3/2.
Arsenic: description
Elemental arsenic occurs in two solid modifications: yellow, and grey or metallic, with specific gravities of 1.97, and 5.73, respectively. The element is a steel grey, very brittle, crystalline, semimetallic (metalloid) solid. It tarnishes in air, and when heated rapidly oxidises to arsenous oxide which has a garlic odour.
Arsenic and its compounds are poisonous as any reader of "who-done-it" books knows. Upon heating arsenic and some minerals containing arsenic, it sublimes (transfers from the solid to the gaseous state, without passing through the liquid state).

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.
Arsenic: physical properties
Density of solid: 5727 kg m-3
Molar volume: 12.95 cm3
Thermal conductivity: 50 W m‑1 K‑1
Arsenic: heat properties
Melting point: 1090 [817 °C (1503 °F)] (under pressure) K
Boiling point: 887 [614 °C (1137 °F)] (sublimes) K
Enthalpy of fusion: 20.5 kJ mol-1
Arsenic: atom sizes
Atomic radius (empirical): 115 pm
Molecular single bond covalent radius: 121 (coordination number 3) ppm
van der Waals radius: 188 ppm
Arsenic: electronegativities
Pauling electronegativity: 2.18 (Pauling units)
Allred Rochow electronegativity: 2.20 (Pauling units)
Mulliken-Jaffe electronegativity: 2.26 (20% s orbital)
Arsenic: orbital properties
First ionisation energy: 944.45 kJ mol‑1
Second ionisation energy: 1793.58 kJ mol‑1
Third ionisation energy: 2735.3 kJ mol‑1
Arsenic: abundances
Universe: 8 ppb by weight
Crustal rocks: 2100 ppb by weight
Human: 50 ppb by weight
Arsenic: crystal structure
Arsenic: biological data
Human abundance by weight: 50 ppb by weight
Arsenic, despite its poisonous reputation, may be a necessary ultratrace element for humans. It is a necessary ultratrace element for red algae, chickens, rats, goats, and pigs. A deficiency results in inhibited growth.
Arsenic: uses
Arsenic: reactions
Reactions of arsenic as the element with air, water, halogens, acids, and bases where known.
Arsenic: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of arsenic where known.
Arsenic: compound properties
Bond strengths; lattice energies of arsenic halides, hydrides, oxides (where known); and reduction potentials where known.
Arsenic: history
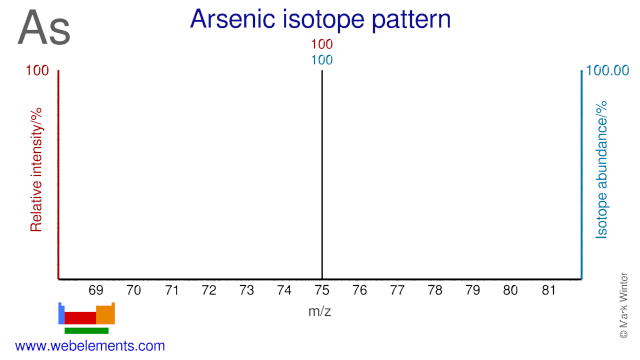
Arsenic was discovered by known since ancient times in unknown at not known. Origin of name: from the Greek word "arsenikon" meaning "yellow orpiment".Arsenic: isotopes
Arsenic: isolation
Isolation: it is not usually necessary to make arsenic in the laboratory as it is commercially available. Arsenic is found in nature in a number of minerals including realgar (As4S4), orpiment (As2S3), arsenolite (As2O3), and iron minerals such as arsenopyrite (FeAsS) and loellingite (FeAs2). Arsenic is made on an industrial scale by heating appropriate minerals in the absence of air. The arsenic is condensed out as a solid.
FeAsS (700°C) → FeS + As(g) → As(s)
